Abstract
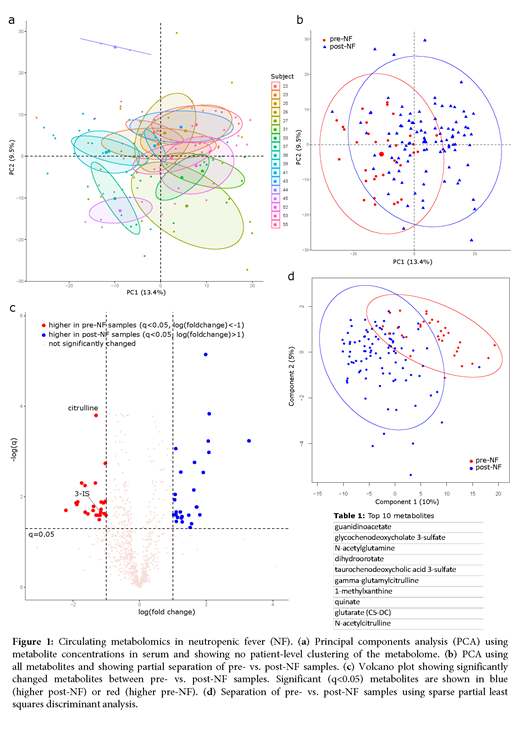
Introduction: Despite antibiotic prophylaxis, most patients with acute myeloid leukemia (AML) develop neutropenic fever (NF) during intensive chemotherapy, suggesting a non-infectious etiology in many cases. In addition, escalated antibiotics used to treat NF increase the risk of Clostridioides difficile infection, promote pathogen colonization, prolong hospitalization, and increase healthcare costs. More effective prevention of NF, preferably using non-antibiotic approaches, is needed. We hypothesized that a longitudinal analysis of the circulating metabolome may reveal novel aspects of NF pathogenesis and identify potential targets for new preventative interventions. Methods: We analyzed 128 longitudinal serum samples from 17 intensively treated adult patients with AML between hospital admission and day 28 of chemotherapy. Samples were collected between 6-8 AM every Mon and Thu. Samples were subjected to ultrahigh performance liquid chromatography-tandem mass spectrometry. Results: All patients developed NF. A total of 1,031 metabolites were identified. Principal components analysis of the circulating metabolome could not resolve individual patients (Fig. 1a). In contrast, pre- vs. post-NF samples were partially clustered (Fig. 1b), suggesting a metabolomic shift associated with NF. After correcting for false discovery, 26 and 27 metabolites were higher in pre- and post-NF samples, respectively (q<0.05, |log fold-change| >1; Fig. 1c). The most significant metabolite that was different between post- and pre-NF samples was citrulline, with a mean concentration ratio of 0.65 between the two groups (q<10-5, Fig. 1c). Citrulline is a known biomarker for total enterocyte mass and its lower levels in post-NF samples indicate intestinal tissue damage as a potential etiology for NF. Another notable metabolite was 3-indoxyl sulfate (3-IS), a tryptophan metabolite and biomarker of gut microbiota diversity and clostridia abundance. 3-IS levels also decreased in post-NF (post vs. pre ratio: 0.45, q=0.02; Fig. 1c), suggesting a protective role for commensal microbiota against NF. Indoles act via the aryl hydrocarbon receptor to repair the intestinal epithelial barrier. Sparse partial least squares discriminant analysis (sPLS-DA) further improved group separation (Fig. 1d). Significantly altered metabolites in the first analysis along with the top 50 metabolites in sPLS-DA were fed into a random forest which generated the final list of 47 metabolites with largest contributions to group separation, including 3-IS and several citrulline metabolites (top 10 metabolites in Table 1). The most frequent metabolites on this list were those in amino acid (n = 17) and lipid (n = 14, including a secondary bile acid) pathways. Conclusions: This first-time analysis of the circulating metabolome in AML patients with NF suggests NF as a metabolic derangement rather than an infectious event in many patients. Augmenting the intestinal epithelium and maintaining a commensal clostridia-rich gut microbiome may help prevent NF. In addition, our list of altered metabolites introduces an unexplored niche for the development of novel, non-antibiotic-based approaches to prevent NF.
Holtan: Incyte: Consultancy, Research Funding; Generon: Consultancy. Weisdorf: Fate Therapeutics: Research Funding; Incyte: Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal